Abstract:
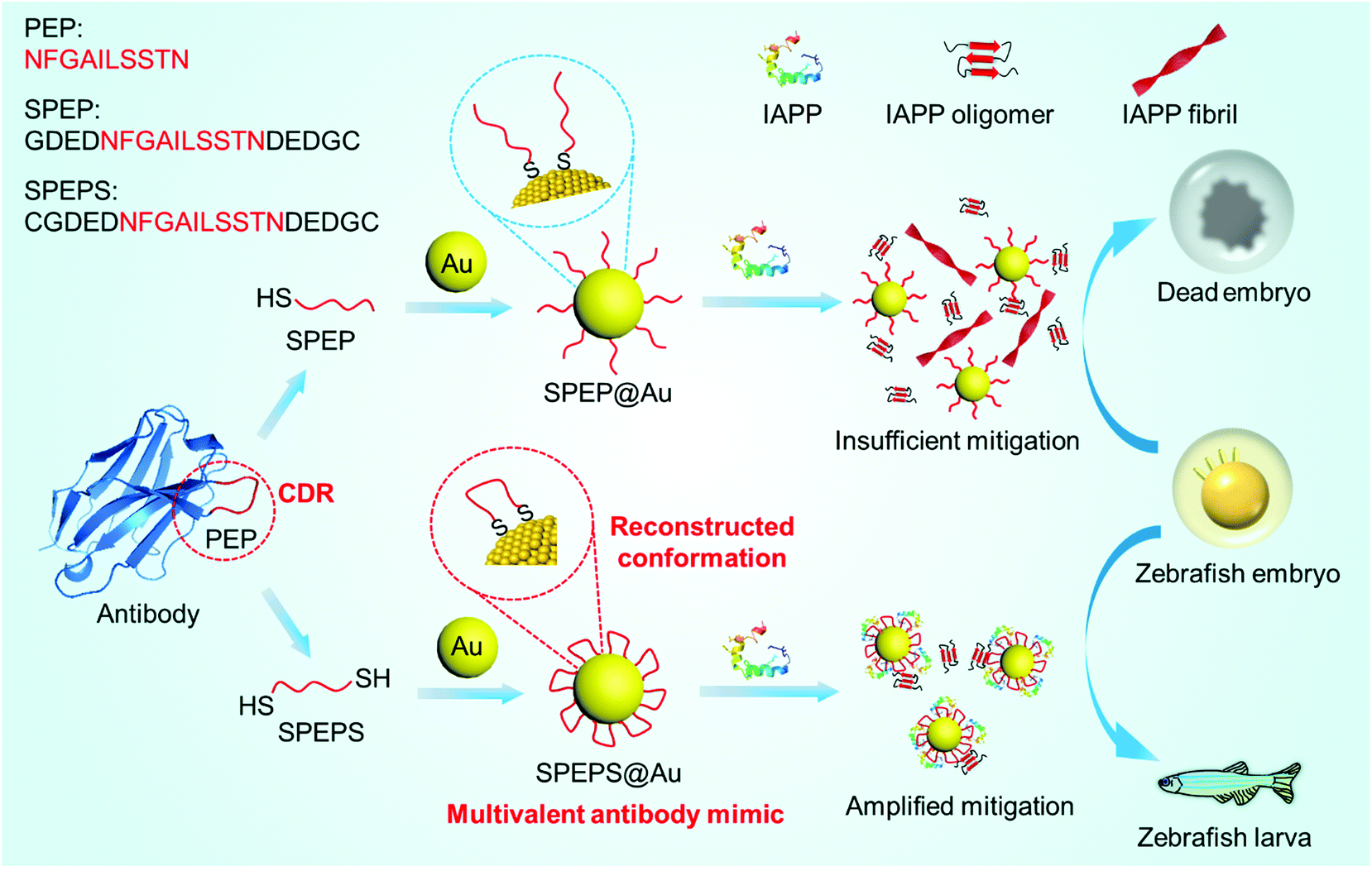
The misfolding and aggregation of human islet amyloid polypeptide (IAPP) into β-sheet-enriched amyloid fibrils is linked to type 2 diabetes. Antibodies are potent inhibitors of IAPP amyloidogenesis, but their preparation is usually complicated and expensive. Here we have created a multivalent antibody mimic SPEPS@Au through conformational engineering of the complementary-determining regions (CDRs) of antibodies on gold nanoparticles (AuNPs). By immobilizing both terminals of an IAPP-recognizing CDR loop (PEP) on the surface of AuNPs, the active conformation of PEP can simply recur on the gold-based antibody mimic, significantly enhancing the binding affinity between PEP and IAPP. SPEPS@Au mitigated amyloidogenesis of IAPP at low sub-stoichiometric concentrations, even after IAPP started aggregating, and dramatically reduced the amyloidogenesis-induced toxicity and ROS production both in vitro and in vivo. The conformation-reconstructed multivalent antibody mimic not only renders a facile strategy to approach potent amyloidogenesis inhibitors, but also provides new perspectives to exploit NP-based substitutes for antibodies in various applications.