Hierarchical Vitalization of Oligotyrosine in Mitigating Islet Amyloid Polypeptide Amyloidogenesis through Multivalent Macromolecules with Conformation-Restrained Nanobody Ligands
- 发表刊物:
- Acs Nano
- 收录刊物:
- SCI
- 卷号:
- 15
- 期号:
- 8
- 页面范围:
- 13319–13328
- ISSN号:
- 1936-0851
- 关键字:
- human islet amyloid polypeptide hierarchical amplification mitigation oligotyrosine conformation-restrained nanobody multivalent effect
- DOI码:
- 10.1021/acsnano.1c03083
- 发表时间:
- 2021-08-24
- 影响因子:
- 15.881
- 摘要:
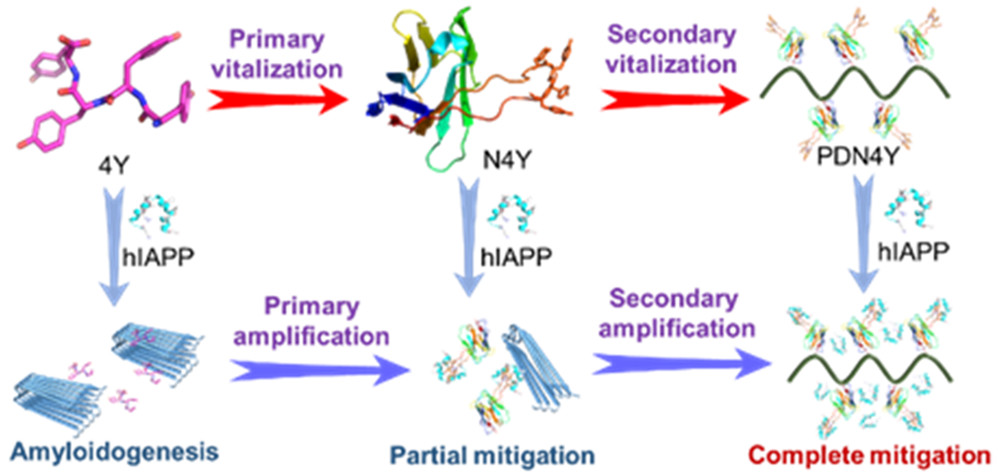
- The development of inhibitors that can effectively mitigate the amyloidogenesis of human islet amyloid polypeptide (hIAPP), which is linked to type II diabetes, remains a great challenge. Oligotyrosines are intriguing candidates in that they can block the hIAPP aggregation through multiplex phenol-hIAPP interactions. However, oligotyrosines containing too many tyrosine units (larger than three) may fail to inhibit amyloidogenesis due to their increased hydrophobicity and strong self-aggregation propensity. In this work, we developed a strategy to hierarchically vitalize oligotyrosines in mitigating hIAPP amyloidogenesis. Tetratyrosine YYYY (4Y) was grafted into the third complementary-determining region (CDR3) of a parent nanobody to construct a sequence-programmed nanobody N4Y, in which the conformation of the grafted 4Y fragment was constrained for a significantly enhanced binding affinity with hIAPP. We next conjugated N4Y to a polymer to approach a secondary vitalization of 4Y through a multivalent effect. The in vitro and in vivo experiments validated that the resulting PDN4Y could completely inhibit the hIAPP amyloidogenesis at low stoichiometric concentrations and effectively suppress the generation of toxic reactive oxygen species and alleviate amyloidogenesis-mediated damage to INS-1 cells and zebrafish (Danio rerio) embryos. The hierarchical vitalization of 4Y via a synergistic conformation restraint and multivalent effect represents a strategic prototype of boosting the efficacy of peptide-based amyloidogenesis inhibitors, especially those with a high hydrophobicity and strong aggregation tendency, which holds great promise for future translational studies.




