·Research Field
1. Proton Exchange Membrane Fuel cells (PEMFCs)/ Electrocatalysis
Low-temperature proton exchange membrane fuel cell is a kind of clean energy form with large-scale commercial production prospect, which has the advantages of high energy density, high conversion efficiency, environmental friendliness and fast startup. At the anode of a PEMFC, hydrogen is oxidized to produce electrons and protons that are transferred to the cathode through an external circuit and the proton exchange membrane, respectively. At the cathode, oxygen is reduced by reaction with protons and electrons to produce water. Because our country is deeply plagued by environmental pollution, there is an urgent need to promote the use of clean energy. Low-temperature fuel cells have also attracted a large amount of investment from electric vehicle manufacturers. Toyota's Mirai oxyhydrogen fuel cell vehicle, which was launched in 2015, features a three-minute inflatable life of 700 kilometers. The oxygen reduction reaction (ORR) is an electrochemical reaction that occurs at the cathode of a fuel cell. The high reaction overpotential requires the noble metal platinum catalyst to proceed the catalytic reaction, which greatly limits the marketization of the fuel cell. On the other hand, the stability of the oxygen reduction catalyst still cannot meet the requirements of the fuel cell market. To solve these problems, we have developed a series of new ORR catalysts. Electrocatalysis is a specific form of catalysis that function at electrode surfaces. The electrocatalyst assists in transferring electrons between the electrode and reactants, and/or facilitates an intermediate chemical transformation described by an overall half-reaction. It is a research hotspot for the energy conversion and storage technologies.
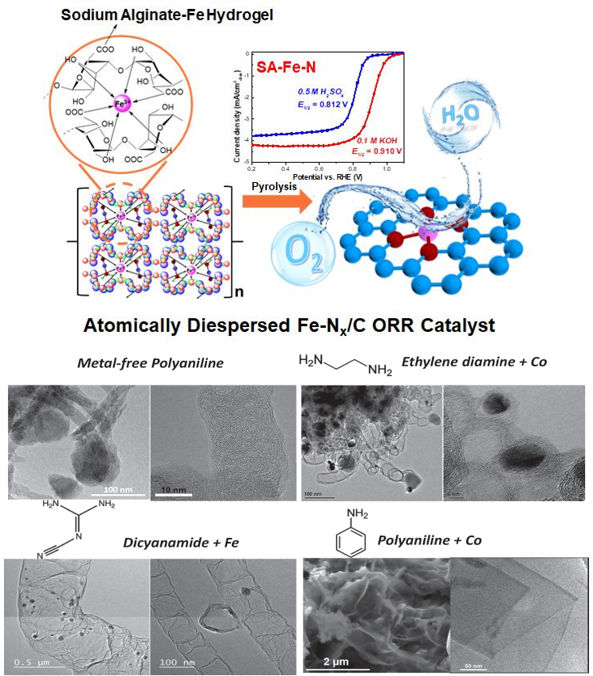
(1) Transition Metal-Nitrogen-Carbon (M-N-C) electrocatalysts for ORR
The successful development of non-precious metal ORR catalysts to replace Pt will undoubtedly facilitate the commercialization of PEMFC. This research is of great significance in both science and industry. Such electrocatalysts are obtained by pyrolyzing transition metal, nitrogen-containing precursor and carbon black at a high temperature (>800°C) and undergoing acid washing and secondary pyrolysis. The active site may be the transition metal-nitrogen-carbon(M-N-C) coordination structure. We focus on the effects of different nitrogen-containing precursor molecules (polyaniline, ethylene diamine, cyanamide, etc.) and transition metals on the carbon structure, the ratio of doped graphite nitrogen/pyridinium nitrogen, and the catalytic activity of the final catalyst. Our group currently has two research priorities: (1) by introducing molecular ligands (such as polymer, macrocyclic compounds) to anchor and protect metal ions from agglomeration during pyrolysis, high activity metal monoatomic M-N-C catalysts are prepared. (2) the geometry and electronic structure of the catalyst are regulated by doping and post-treatment, thereby improving the stability of the M-N-C catalyst in fuel cell operation.
Representative publications: Adv. Energy Mater., 2018, 8, 1801226; Adv. Energy Mater., 2014, 4, 1301415; Adv. Mater. 2014, 26, 1378; ACS Catal. 2014, 4, 3193; Adv. Sci. 2016, 3, 1600140.
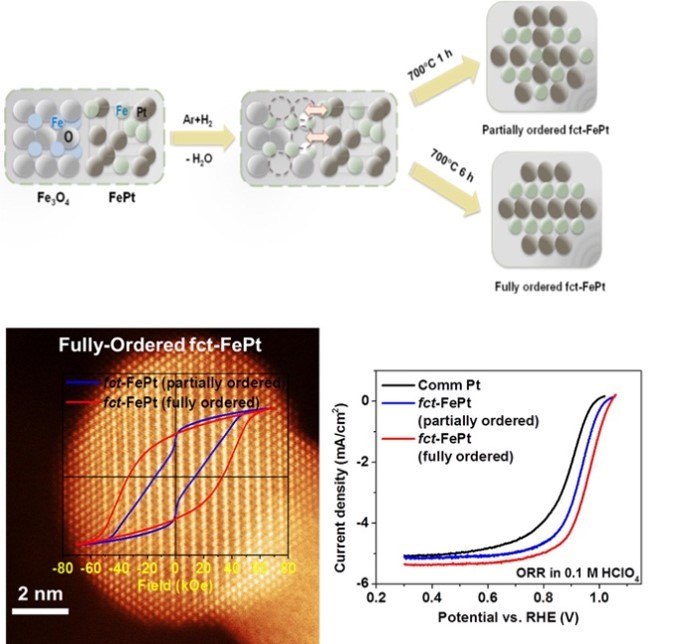
(2) Structurally-Ordered PtM (M = transition metal) Nanoparticles for ORR
How to reduce the amount of Pt in the ORR catalyst and increase its activity is also an important direction in the research of fuel cell electrocatalysis. Although the ORR catalytic activity of ordinary PtM (M = Co, Ni, Fe, etc.) can be improved by regulating the position of the d electron center, its M is easily etched in acidic environment, causing destruction of the catalyst structure. We have found that the degree of crystal ordering of Pt-based nanocrystals has an important influence on the activity and stability of the catalysts. For instance, we report that the 8 nm fully-ordered L10-FePt NPs can be synthesized using dumbell FePt-Fe3O4 NPs to introduce atomic defects during reductive annealing. They exhibit excellent activity (much higher than disordered fcc-FePt and Pt/C) and stability (no Fe loss in acid after 20000 cycles accelerated potential scanning). Our group has two research directions: (1) prepare various chemically ordered PtM or PtM1M2 with different sizes and morphologies by rational design of nanoprecursors and post-treatments; (2) improve their stability in fuel cell operation.
Representative publications: Nano Lett. 2015, 15, 2468;Nano Energy, 2016, 29, 178;Angew. Chem. Int. Ed., 2015, 127, 7634.
1. Electrochemical CO2 Reduction and Water Splitting (Hydrogen Evolution and Oxygen Evolution)
In recent years, with the excessive exploitation of fossil fuels, the increase of CO2 emissions has been a serious threat to the ecological environment. Converting CO2 into other high value-added chemicals can be used not only reduce carbon emissions, but also enable the reuse of resources. Electrochemical reduction can realize the reduction and conversion of CO2 under mild conditions. The reaction process is controllable and simple, which can be carried out at normal temperature and pressure, moreover, the reaction product is green and cyclable, which has attracted extensive attention in academia and industry. Hydrogen Evolution Reaction (HER) is a cheap and efficient way to produce hydrogen, which can provide hydrogen energy for green energy forms such as fuel cells. However, its efficiency and cost are constrained by the use of Pt catalysts and the large overpotential of the oxygen evolution reaction (OER) on the anode. Besides, the OER and CO2 reduction coupling can constitute an artificial photosynthesis device. In summary, the development of inexpensive and efficient CO2 reduction, hydrogen evolution and oxygen evolution catalysts are of great significance for green energy and environmental protection.
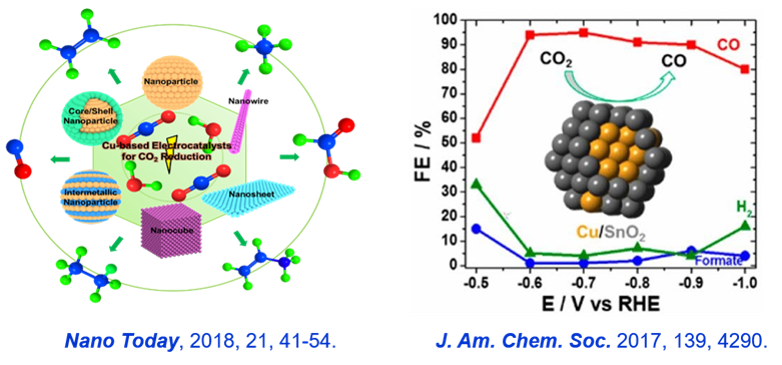
(1) Development of catalysts and devices for the production of chemicals by CO2 reduction.
CO2 reduction is a multi-step reaction. The products usually contain CO, CH4, C2H4, C2H6, HCOOH, C2H5OH etc., involving multiple electrons and protons, which are slow in kinetics and complicated in mechanism. Our group mainly studies: (1) the structural regulation of Cu-based CO2 reduction catalysts to prepare organic compounds such as alkane and olefin; (2) the design of CO2 reduction catalysts to produce CO and formic acid; (3) the construction of high-efficiency reaction system.
Representative publications: J. Am. Chem. Soc. 2017, 139, 4290;Nano Today, 2018, 21, 41-54; Nano Energy 2016, 24, 1.
(2) Development of hydrogen evolution and oxygen evolution catalysts and electrolyzed water devices
Our current research focuses on oxygen evolution reaction: (1) structural design and performance optimization of non-precious metal catalysts such as multi-metal hydroxy oxidants; (2) structural change analysis of catalysts in OER process; (3) the construction of overall water splitting and artificial photosynthesis devices. As for hydrogen evolution reaction: (1) development of non-Pt catalysts such as metal phosphide sulfides; (2) structural design and performance control of catalysts.
Representative publications: Adv. Mater., 2018, 30, 1800757;J. Am. Chem. Soc. 2015, 137, 7071; Nano Energy 2017, 42, 69; J. Mater. Chem. A 2017, 5, 25378.

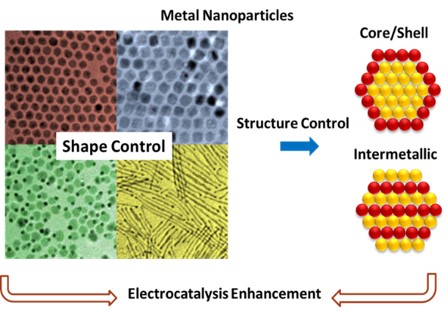
3. Preparation and Characterizations of Multi-Metal Nano-catalysts
Due to their special electronic structure, large area/volume ratio, and easy-to-regulate morphology/surface crystal surface, nanocatalysts have received great attention in many fields such as catalysis, energy storage and sensing. However, the preparation of metal nanocatalysts of specific composition, size, morphology and structure required by different synthetic methods is a great challenge for the synthesis researcher. Our group has realized the controllable preparation of multi-metal nano-catalysts by various synthetic methods, and further realized the structural regulation of the catalyst through post-treatment, thus developing a catalyst suitable for various catalytic reactions.
Representative publications: Nano Energy 2016, 29, 178; Nano Today 2014, 9, 668.
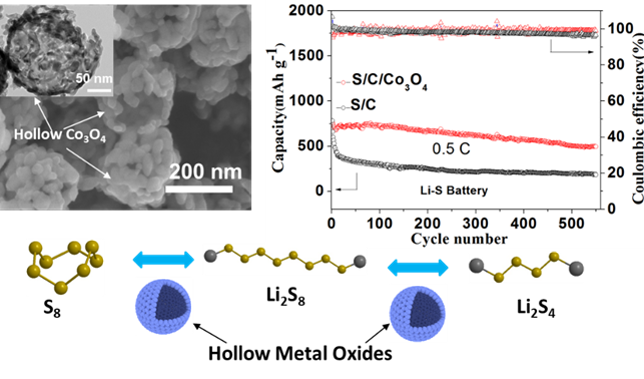
4. Advanced Secondary Batteries (lithium/sodium battery)
The explosive growth in human demand in the three areas of consumer electronics, automotive and grid storage has exposed traditional lithium-ion batteries in terms of energy density, rate performance and safety. Therefore, the development of secondary batteries, such as metal-air batteries, lithium-sulfur batteries, sodium-ion batteries, etc. is the focus of current research in electrochemical energy. Lithium (sodium) battery is a type of battery that is made of a lithium (sodium) metal or a lithium (sodium) alloy as a negative electrode material. During charge and discharge, lithium ions intercalate and escape between the two electrodes, and migrate back and forth between the positive and negative electrodes, thereby causing the system to generate current. Lithium-sulfur battery is a kind of energy storage technology that has received extensive attention in recent years. It usually uses sulfur as the positive electrode and lithium as the negative electrode, which has high energy density and low cost. Sulfur is one of the most abundant elements in nature, with a theoretical specific capacity of 1675 mA h / g (the theoretical specific capacity of lithium cobalt oxide in lithium ion batteries is only 280 mA h / g), which is composed of metallic lithium. The lithium-sulfur battery system has a theoretical mass energy density of up to 2600 W h/kg, far exceeding that of lead-acid batteries, sodium-sulfur batteries and lithium-ion batteries, and has a large potential for use in large-scale energy storage. However, the lower conductivity of sulfur and the volume effect during cycling inhibit its capacity and significantly degrade the capacity of the battery during cycling. Another important issue is the shuttle effect of the intermediate product polysulfide during the cycle: the discharge process of sulfur is carried out in multiple steps, and the sulfur and the final discharge product Li2S/Li2S2 are insoluble in the electrolyte, while the intermediate product Li2Sx (4 ≤ x ≤ 8) in the charging and discharging process is dissolved in the electrolyte and diffused to the negative electrode.After the negative electrode is reduced by metal lithium, the product diffuses back to the positive electrode to be oxidized, thereby shuttle back and forth between the positive and negative electrodes, causing corrosion of the negative electrode and bringing irreversible loss of active material and capacity, which makes the battery cycle life significantly lower. The working principle of the sodium ion battery is similar to that of the lithium ion battery, and the sodium ion is used to insert and discharge between the positive and negative electrodes to achieve charge and discharge. Compared with lithium-ion batteries, sodium-ion batteries have the following advantages: (1) sodium salt raw material is abundant in reserves and low in price; (2) due to sodium salt characteristics ,low-concentration electrolyte is allowed (The same concentration of electrolyte, sodium salt conductivity is higher than about 20% of lithium electrolyte) to reduce the cost; (3) sodium ion does not form alloy with aluminum, and the negative electrode can use aluminum foil as the current collector, which can further reduce the cost by about 8% and reduce the weight by about 10%; (4) The sodium ion battery has no overdischarge characteristics, allowing the sodium ion battery discharges to zero volt. The energy density of sodium ion battery is more than 100Wh/kg, which is comparable to lithium iron phosphate battery. Due to its obvious low cost, it is expected to be applied in large-scale energy storage. Our main research directions in the field of electrochemical energy storage are: (1) the application of metal nanocatalysts in the catalytic conversion of polysulfide ions in lithium-sulfur batteries; (2) the electrode material design of sodium ion batteries; (3)the optimization of positive ternary materials for lithium ion batteries.
Representative publications: Nanoscale, 2018, 10, 5634; Electrochimica Acta 2018, 284, 526; Electrochimica Acta 2018, 282, 973.