Supervisor of Master's Candidates
Gender:Male
Status:Employed
Department:School of Life Science and Technology
Education Level:Postgraduate (Doctoral)
Degree:Doctoral Degree in Science
Discipline:Biomedical Engineering
Paper Publications
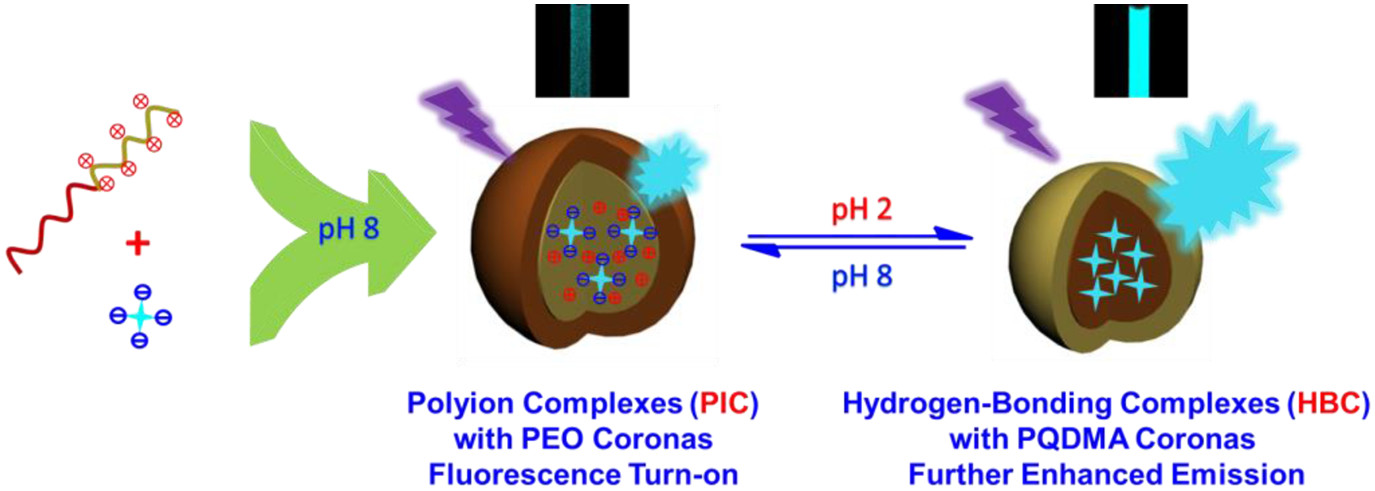
pH-Regulated Reversible Transition Between Polyion Complexes (PIC) and Hydrogen-Bonding Complexes (HBC) with Tunable Aggregation-Induced Emission
Indexed by:Journal paper
Journal:ACS Applied Materials & Interfaces
Volume:8
Issue:6
Page Number:3693–3702
Key Words:supramolecular interaction polyion complex hydrogen bonding complex pH-responsive schizophrenic micelle aggregation-induced emission
DOI number:10.1021/acsami.5b08970
Date of Publication:2015-11-19
Impact Factor:10.383
Abstract:The mimicking of biological supramolecular interactions and their mutual transitions to fabricate intelligent artificial systems has been of increasing interest. Herein, we report the fabrication of supramolecular micellar nanoparticles consisting of quaternized poly(ethylene oxide)-b-poly(2-dimethylaminoethyl methacrylate) (PEO-b-PQDMA) and tetrakis(4-carboxylmethoxyphenyl)ethene (TPE-4COOH), which was capable of reversible transition between polyion complexes (PIC) and hydrogen bonding complexes (HBC) with tunable aggregation-induced emission (AIE) mediated by solution pH. At pH 8, TPE-4COOH chromophores can be directly dissolved in aqueous milieu without evident fluorescence emission. However, upon mixing with PEO-b-PQDMA, polyion complexes were formed by taking advantage of electrostatic interaction between carboxylate anions and quaternary ammonium cations and the most compact PIC micelles were achieved at the isoelectric point (i.e., [QDMA]/[COO+–] = 1), as confirmed by dynamic light scattering (DLS) measurement. Simultaneously, fluorescence spectroscopy revealed an evident emission turn-on and the maximum fluorescence intensity was observed near the isoelectric point due to the restriction of intramolecular rotation of TPE moieties within the PIC cores. The kinetic study supported a micelle fusion/fission mechanism on the formation of PIC micelles at varying charge ratios, exhibiting a quick time constant (τ1) relating to the formation of quasi-equilibrium micelles and a slow time constant (τ2) corresponding to the formation of final equilibrium micelles. Upon deceasing the pH of PIC micelles from 8 to 2 at the [QDMA]/[COO+–] molar ratio of 1, TPE-4COOH chromophores became gradually protonated and hydrophobic. The size of micellar nanoparticles underwent a remarkable decrease, whereas the fluorescence intensity exhibited a further increase by approximately 7.35-fold, presumably because of the formation of HBC micelles comprising cationic PQDMA coronas and PEO/TPE-4COOH hydrogen-bonded cores, an inverted micellar structures compared to initial PIC micelles. Moreover, the pH-mediated schizophrenic micellar transition from PIC to HBC with tunable AIE characteristic was reversible.
Links to published journals:https://doi.org/10.1021/acsami.5b08970
